
THE INNOVATIVE HYBRID COBALT-CHROMIUM ABLUMINAL BIODEGRADABLE POLYMER DES
BioMatrix™ Alpha presents the best in class stent platform design with unique pro-healing coating from the pioneer in abluminal biodegradable technology3.
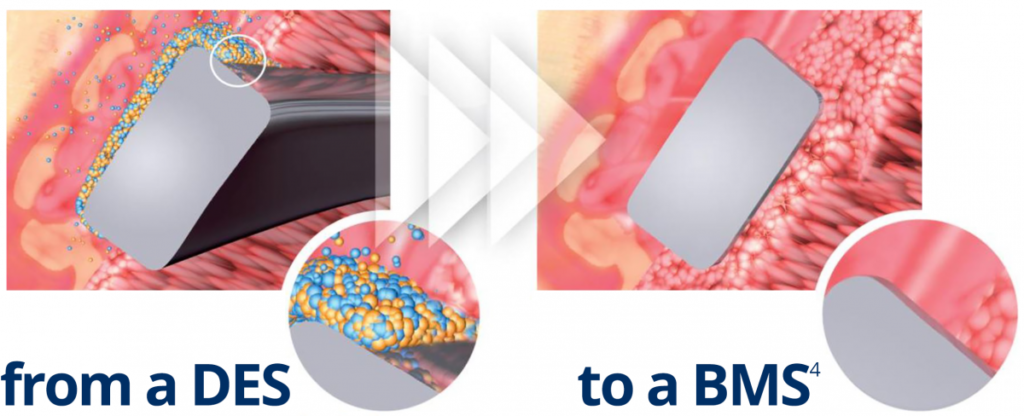
Abluminal coating absorbed after 6 to 9 months4
No drug carrier or drug inside the stent
- Early BMS-like endothelial coverage2
- More targeted drug release
- Reduced systemic exposure
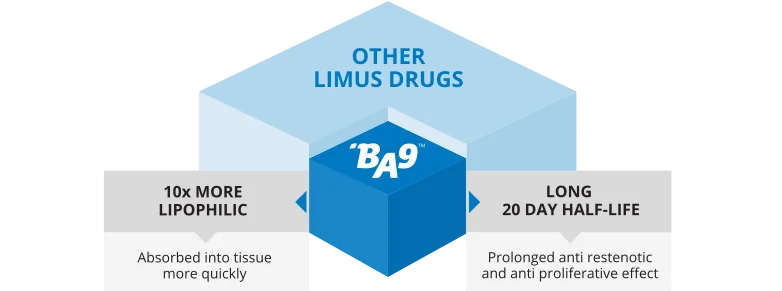
BA9™ DESIGNED SPECIFICALLY FOR CORONARY STENT APPLICATION

- Unique drug and proprietary of Biosensors International Group, Ltd.
- Designed for properties that would support healing and re-endothelialization
- Chemical characteristics allow:
- – delivered with synchronous absorption of proprietary PLA polymer
- – with no loss to the systemic system.
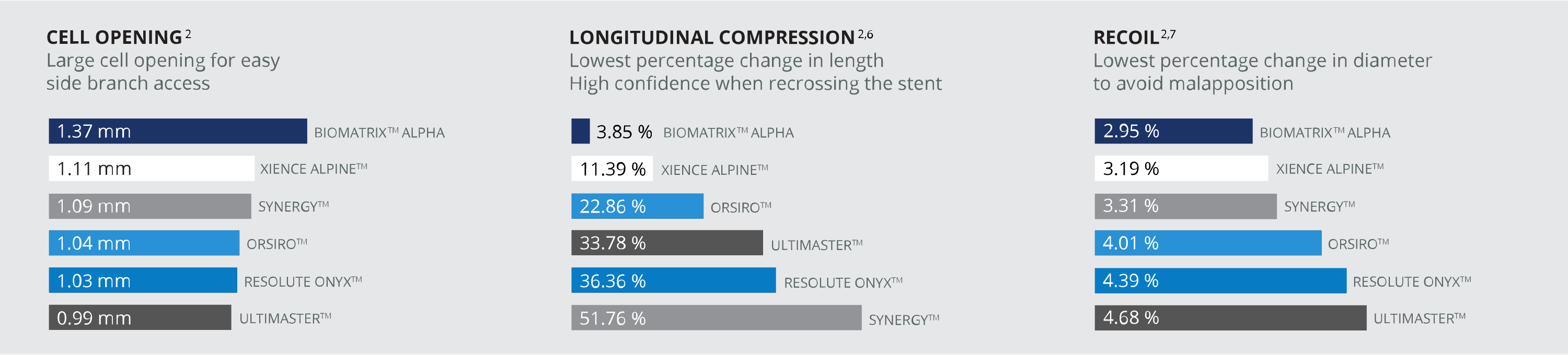
ALPHA BY DESIGN BEST IN CLASS PERFORMANCE VS OTHER STENTS2
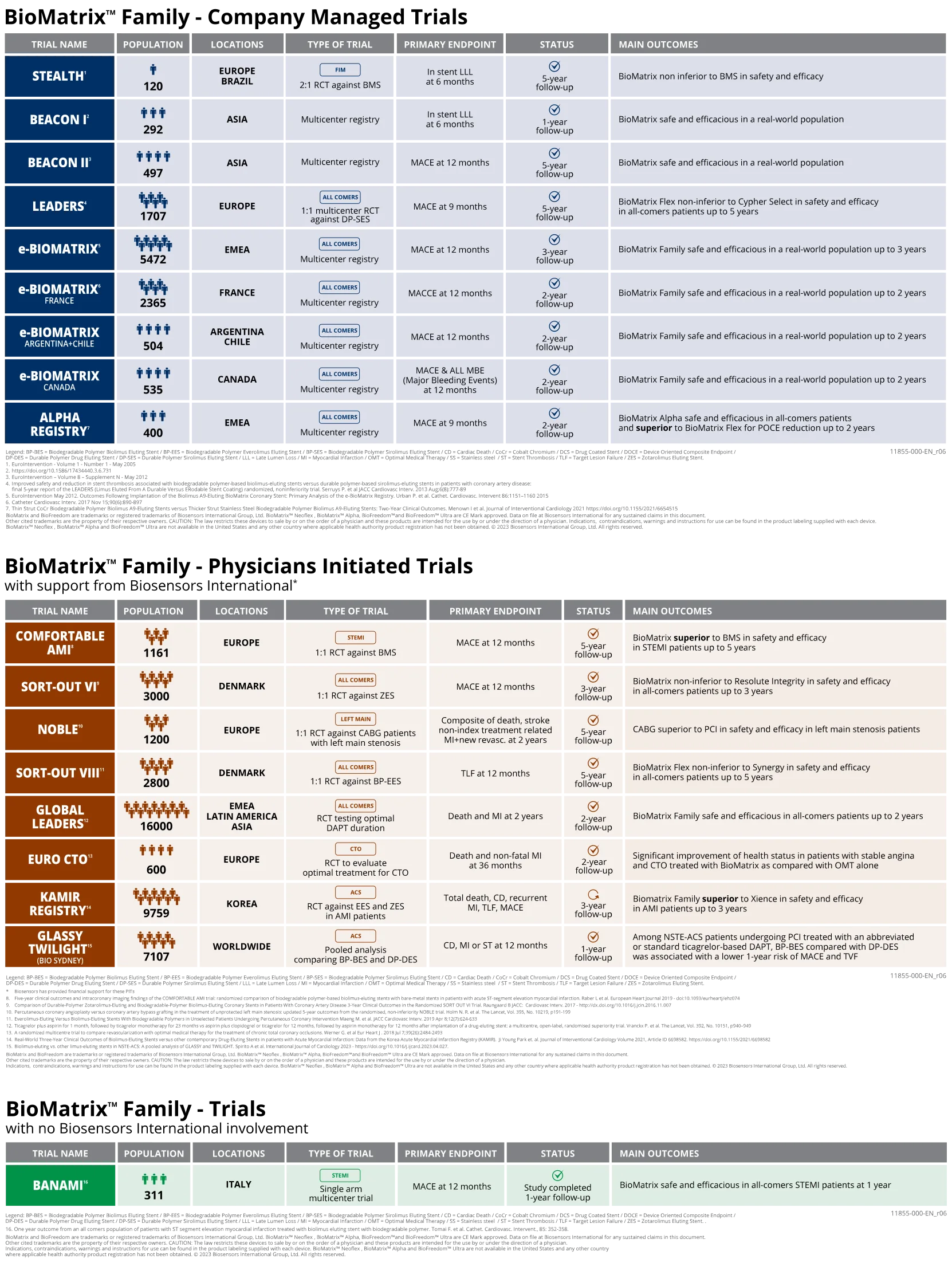
BIOMATRIX™ FAMILY CLINICAL PROGRAM
Proven safety of the Biolimus A9™ (BA9™) drug
BA9™ DESIGNED SPECIFICALLY FOR CORONARY STENT APPLICATION
Bridging science and innovative technology
BA9™ Designed for Vascular Stent Technologies, optimized for local drug delivery
BA9™ Designed for Vascular Stent Technologies, optimized for local drug delivery
12244-000-EN, 11521-000-EN, 11463-000-EN - Rev.01 + 11881-000-EN - Rev.04
BIOMATRIX™ FAMILY CLINICAL PROGRAM

Proven safety of a DES
with an abluminal biodegradable polymer
INNOVATIVE TECHNOLOGY FOR OPTIMIZED ARTERIAL HEALING
The BioMatrix™ family favourably influences local wound healing processes.
Highly localised delivery of anti-restenotic BA9™ for at least six months.
Degradation of specifically designed PLA polymer to lactate, which has the potential to facilitate wound healing.
SPECIFICALLY DESIGNED PRO-HEALING POLYMER
NOT ALL POLYMERS ARE THE SAME
- Biosensors’ PLA polymer degrades to naturally occuring Lactic Acid and Lactate
- Lactate plays a key role in local arterial wound healing processes, mainly via enhanced Vascular Endothelial Growth Factor (VEGF) production6,7
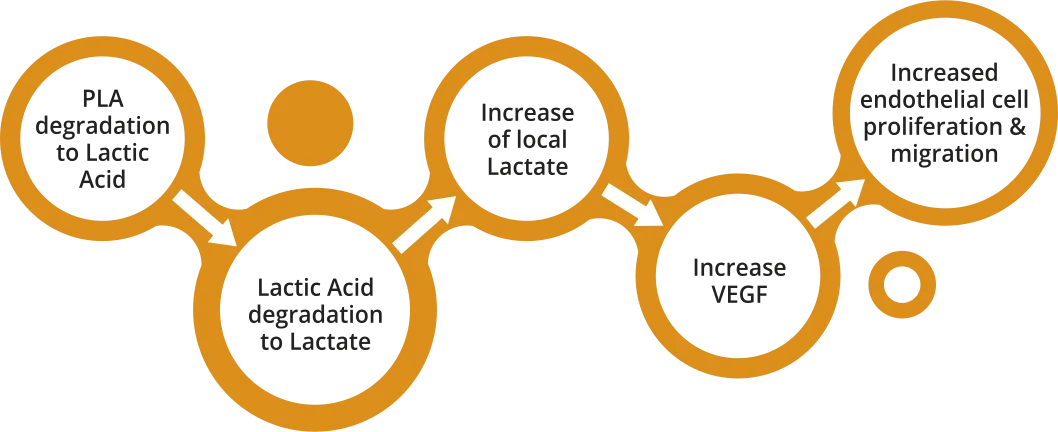
The localized presence of PLA-derived lactate has the potential to facilitate arterial wound healing, including re-endothelialization6-7
12244-000-EN + 11447-000-EN + 11582-000-EN - Rev.01 + 11881-000-EN - Rev.04
BIOMATRIX™ FAMILY CLINICAL PROGRAM

Stent platform
UNCOMPROMISED PERFORMANCE

12201-001-EN - Rev.01 + 11881-000-EN - Rev.04
BIOMATRIX™ FAMILY CLINICAL PROGRAM

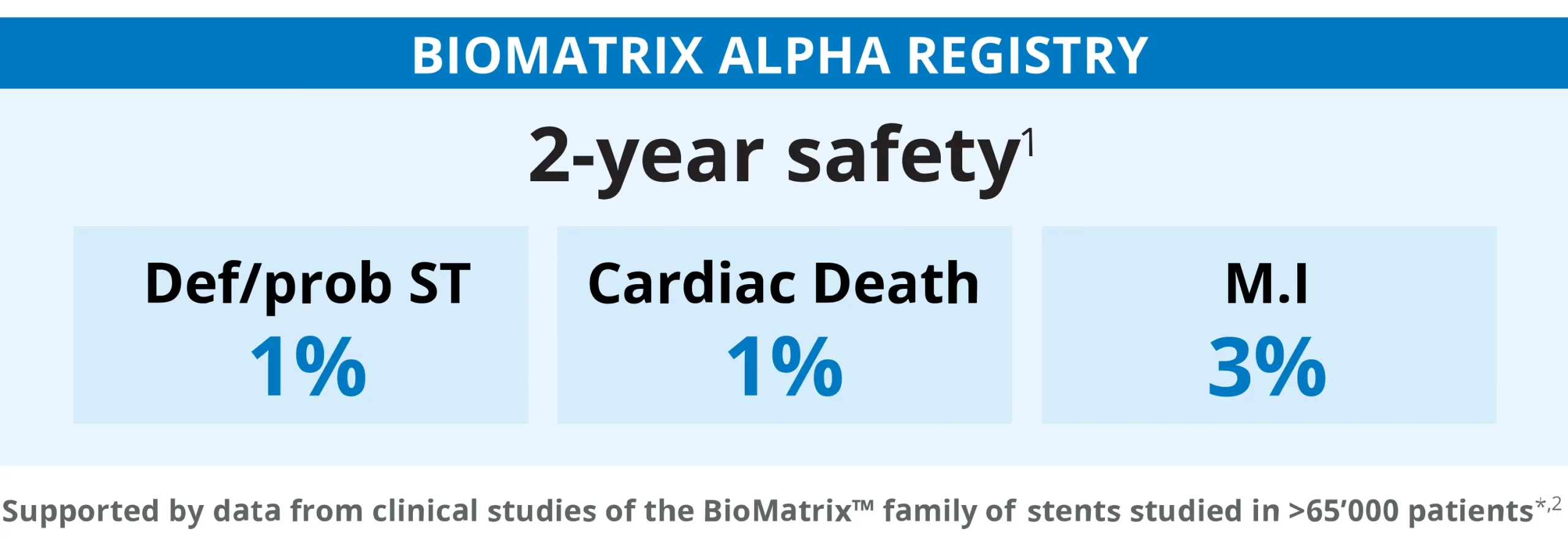
THE BIOMATRIX ALPHA REGISTRY1 FIRST CLINICAL EVIDENCE CHARACTERIZING SAFETY AND EFFICACY OF THE NEW COCR BIOLIMUS-A9 ELUTING STENT
Results at 9 months
Results at 24 months
LEADERS2 THE FIRST ALL COMERS TRIAL OF PCI
(LIMUS ELUTED FROM A DURABLE VERSUS ERODABLE STENT COATING)
Results:
Clinical benefit shown in patients undergoing complex PCI2
E-BIOMATRIX3
Results 3 years4:
COMFORTABLE AMI 2 YEAR FOLLOW-UP5
Thus, BioMatrix has become the Gold Standard in biodegradable technology.
12244-000-EN, 11140-000-EN - Rev.01 + 11134-000-EN, 11136-000-EN - Rev.02 + 11881-000-EN - Rev.04
CAUTION: Please note that the following pages are exclusively reserved for Health Care Professionals in countries with applicable health authority product registrations. To the extent this site contains information intended for use by licensed medical professionals, such materials are not intended to offer professional medical advice. Prior to use, please consult device labeling for prescriptive information and operating instructions. Please contact your Biosensors International representative for availability or the products and registration status.
The law restricts these devices to sale by or on the order of a physician. Prior to use, it is important to read the “Instructions for Use” supplied with these devices for indications, contraindications, suggested procedures, warnings, and precautions.
Biosensors’ interventional cardiology products, including BioMatrix NeoFlex™, BioMatrix™ Alpha, BioFreedom™, BioFreedom™ Ultra, BMX-J® and RISE™ NC, are not available for sale in the United States and certain other countries. ALLEGRA™ is a product of NVT GmbH. Blue Sail Medical Co., Ltd is the ultimate parent company of NVT GmbH and Biosensors International Group, Ltd. and its subsidiaries are collaborating for the commercialization of the ALLEGRA™ device.
BioMatrix NeoFlex, BioMatrix Alpha, BioFreedom, BioFreedom Ultra, BMX-J, Juno, S-Stent and Rise NC are trademarks or registered trademarks of Biosensors International Group, Ltd. ALLEGRA is a trademark or registered trademark of NVT AG. All other cited trademarks are the property of their respective owners.
