WHAT IS BIOFREEDOM™?
BioFreedom™ is a polymer- and carrier-free Drug Coated Stent with BA9™ (DCS).
The combination of a selectively micro-structured abluminal surface (SMS) and Biolimus A9™ make BioFreedom™ a unique stent.
At least 40%1 of PCI patients are High Bleeding Risk (HBR) requiring an individualized approach, BioFreedom™ provides a more appropriate treatment strategy.
The rapid 28 day transfer of BA9™ to the coronary artery and rapid reendothelialization of the stented vessel, make BioFreedom™ the most relevant choice of stent for High Bleeding Risk (HBR) patients who cannot tolerate long dual antiplatelet therapy (DAPT).
The LEADERS FREE trial proved superior safety and efficacy of the BioFreedom™ DCS vs a BMS in the previously understudied and underserved HBR patient population.2
BioFreedom™ is now listed as stent of choice in ESC DAPT guidelines, for 1-month ultra-short DAPT in patients with stable CAD in whom longer DAPT regimes poses safety concerns.
11524-000-EN - Rev.01 + 11881-000-EN - Rev.04
1. Ueki et al. Validation of Bleeding Risk Criteria (ARC-HBR) in Patients Undergoing Percutaneous Coronary Intervention and Comparison with Contemporary Bleeding Risk Scores. EuroIntervention. 2020 Feb 18. DOI: 10.4244/EIJ-D-20-00052
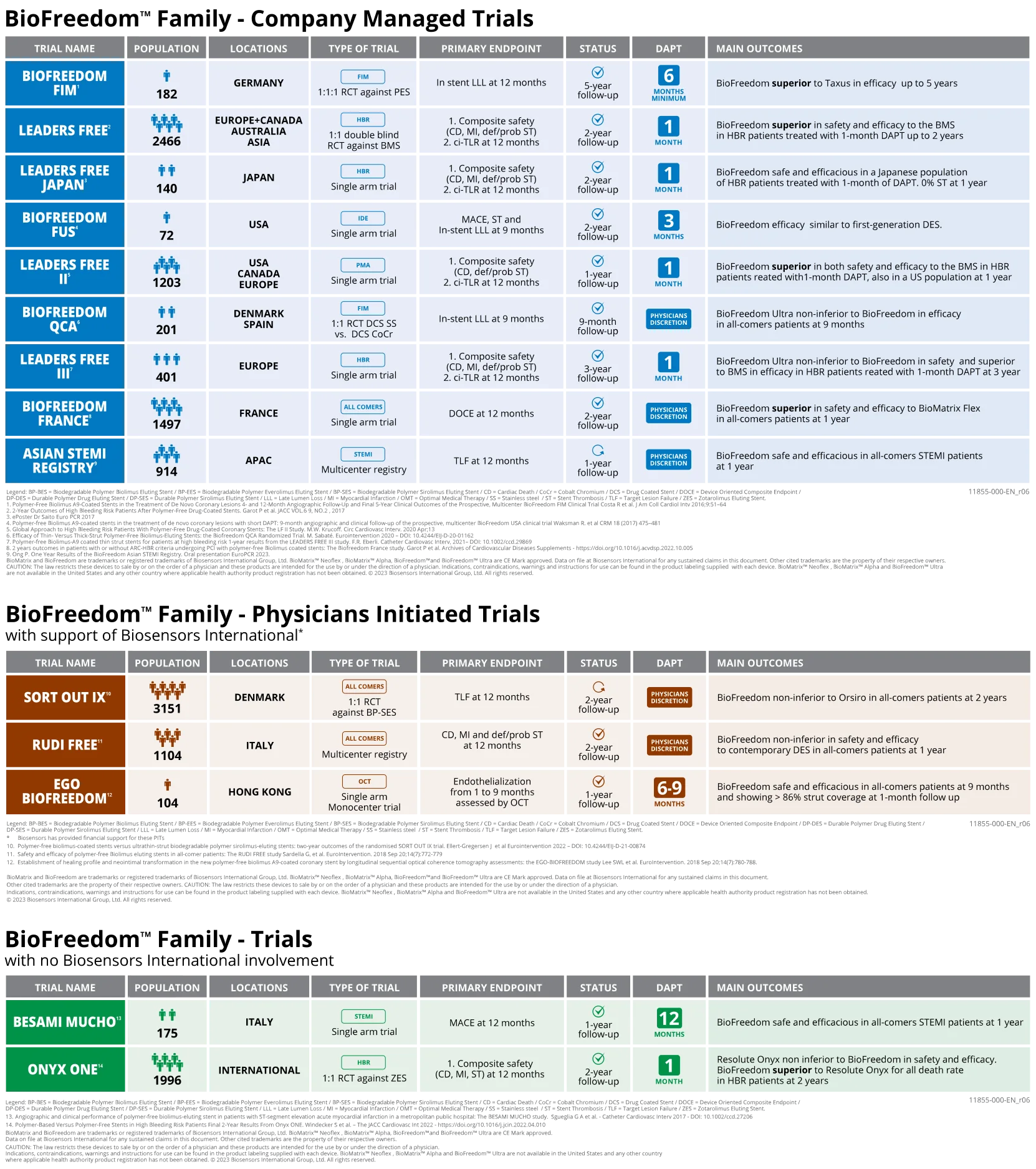
BIOFREEDOM™ FAMILY CLINICAL PROGRAM
BA9™ (Biolimus A9™)
Designed for vascular stent technologies
BA9™, AN EFFECTIVE CYTOSTATIC LIMUS DRUG
- Greater Local Bioavailability
- Targeted Drug Release
- Local tissue warehousing allowing Sustained Tissue Release, with Therapeutic Effect up to 100 days12,13
- Longer half-life than other commonly used Limus Drugs, approximately 20 days in tissue12,13
- Potent Neointimal Suppression
INCREASING SAFETY AND EFFICACY
By leaving a bare metal stent luminal surface, BioFreedom™ promotes rapid reendothelialization, improves the healing process14 and allows for ultra-short 1 month DAPT.
11521-000-EN - Rev.01 + 11903-000-EN - Rev.01 + 11524-000-EN - Rev.01 + 11881-000-EN - Rev.04
Selectively Micro-Structured Surface (SMS)
BIOFREEDOM™ FAMILY CLINICAL PROGRAM

Meeting the need of
High Bleeding Risk (HBR) Patients
At least 40% of PCI patients are High Bleeding Risk (HBR) where there is a need to avoid prolonged dual antiplatelet therapy (DAPT).1-9
The BioFreedom™ Drug-Coated Stent (DCS) is significantly safer and significantly more efficacious than a BMS in High Bleeding Risk patients, with a DAPT regime of only one month, the shortest DAPT regime mandated for all patients in a published, double-blind randomized controlled trial.10
By directly delivering BA9™ – an effective anti-restenotic therapy – without polymer or carrier, becoming a BMS at 28 days, the DAPT regime can be shortened when treating patients with the BioFreedom™ stent.10,11

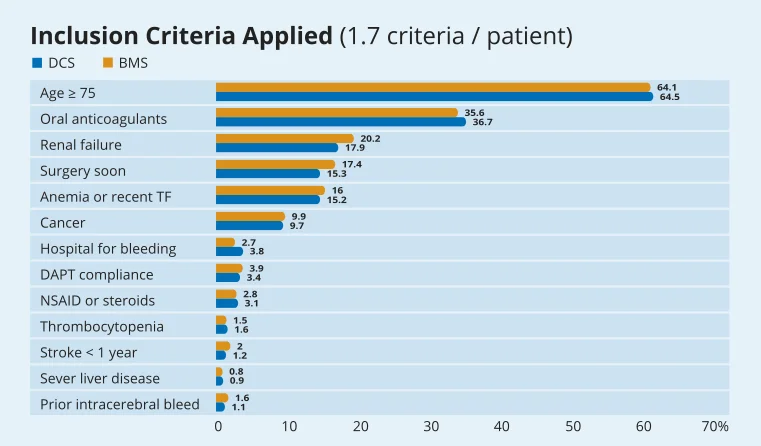
In LEADERS FREE, High bleeding risk patients include, but are not exhaustively: Age >75 OACs, Renal Failure, planned surgery within the next 12 months, anemia of recent TF, Cancer, hospitalisation for bleeding, poor DAPT compliance, NSAIDS steroids, thrombocytopenia, stroke <1 year, severe liver disease, prior intracerebral bleed.
BioFreedom™ is now listed as stent of choice in ESC DAPT guidelines, for 1 month-ultra short DAPT in patients with stable CAD in whom longer DAPT regimes poses safety concerns*.
11523-000-EN - Rev.01 + 11524-000-EN - Rev.01 + 11881-000-EN - Rev.04
*European Heart Journal (2018)39, 213-254
1. Rao et al. AHJ 2013;166:273-281.e4
2. Rittger H et al. Herz 2014;39(2):212-8
3. Faxon et al. Circ Cardiovasc Interv 2011;4:522-34
4. De Biase et al. Transl Med 2015;11(3):14-23
5. To et al. Circ Cardiovasc Interv-2009;2:213-21
6. Wiviott et al. NEJM 2007;357:2001-15
7. Pilgrim et al. Circ Cardiovasc Interv. 2012;5:202-210
8. Shanmugam VB et al. Journal of Geriatric Cardiology 2015;12:174−184
9. Urban P. et al. Am Heart J 2013;165:704-9
10. Urban P et al. N Engl J Med 2015;373:2038-47
11. Ueki et al. Validation of Bleeding Risk Criteria (ARC-HBR) in Patients Undergoing Percutaneous Coronary Intervention and Comparison with Contemporary Bleeding Risk Scores. EuroIntervention. 2020 Feb 18. doi: 10.4244/EIJ-D-20-00052
BIOFREEDOM™ FAMILY CLINICAL PROGRAM

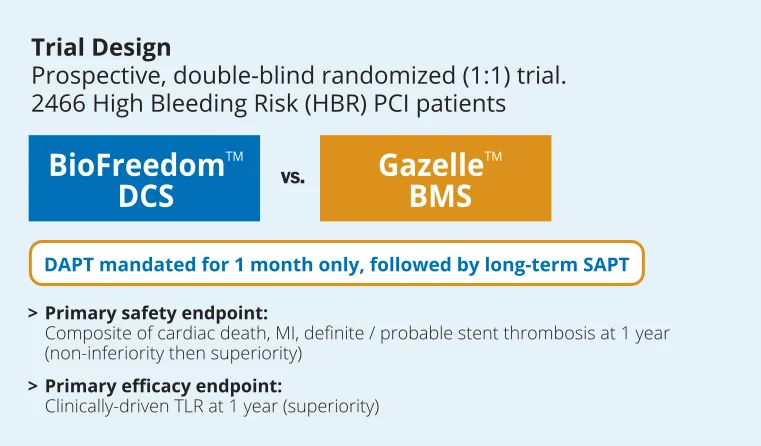
LEADERS FREE
WITH LEADERS FREE, BIOFREEDOM™ BECOMES THE STANDARD OF CARE FOR HIGH BLEEDING RISK (HBR) PATIENTS
LEADERS FREE is a prospective, double-blind, randomized (1:1) clinical trial comparing the BioFreedom™ drug coated stent (DCS) to the Gazelle™ bare metal stent (BMS) in 2466 High Bleeding Risk (HBR) patients with 1 month dual anti-platelet therapy (DAPT).
The results demonstrated that BioFreedom™ is superior to a bare metal stent with respect to the primary safety and efficacy endpoints at 1 year when used with a 1-month course of DAPT11.
LEADERS FREE is currently the only published trial to exclusively enroll HBR patients with 1 month DAPT.
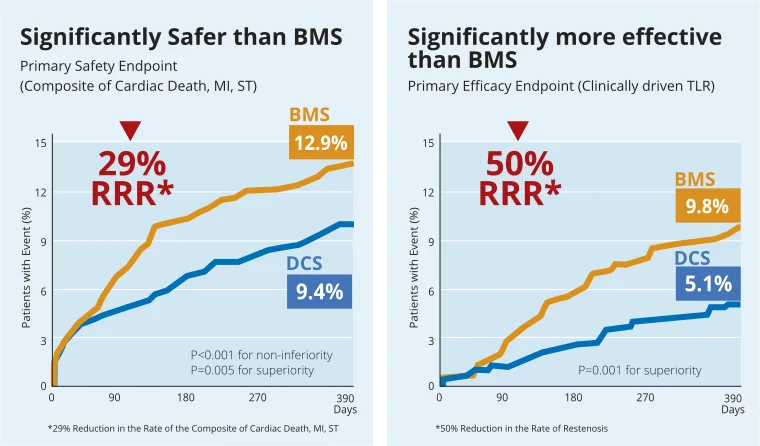
LEADERS FREE, at 1 year proved that BioFreedom™ is the only active stent with 1 month DAPT that demonstrated significantly superior outcomes to BMS in High Bleeding Risk Patients11. NEJM (October 2015)
LEADERS FREE 2 year follow-up maintains that BioFreedom™ and 1 month DAPT followed by SAPT alone should be the treatment strategy of choice for HBR patients undergoing PCI. JACC (January 2017)
BioFreedom™ is now listed as stent of choice in ESC DAPT guidelines, for 1 month-ultra short DAPT in patients with stable CAD in whom longer DAPT regimes poses safety concerns12
Rationale and Design of the LEADERS FREE Trial
A Randomized Double-Blind Comparison of the BioFreedom Drug-Coated Stent vs the Gazelle Bare Metal Stent in Patients at High Bleeding Risk Using a Short (1 Month) Course of Dual Antiplatelet Therapy Urban p. Am Heart J. 2013 May;165(5):704-9
1. LEADERS FREE is the first randomized clinical trial dedicated to HBR patients who received 1 month of DAPT followed by single antiplatelet therapy.
2. Such patients are often excluded from stent and drug trials, constitute a rapidly growing proportion of PCI candidates and suffer high event rates.
3. Together with an ultra short one-month only DAPT course, the use of BioFreedom™ (a Biolimus A9™ polymer and carrier free DCS) was both significantly safer and more effective than a control BMS in HBR patients.
The pre-specified Acute Coronary Syndrome sub-group of the LEADERS FREE trial was presented as a late breaking clinical trial at Euro PCR 2016 by Dr Christoph K. Naber and published13.
This sub-group analysis reinforces the benefit of the BioFreedom™ DCS vs BMS in HBR patients. The improvement in safety and efficacy achieved with BioFreedom™ is even greater in the high risk HBR ACS patient population.

In HBR patients with ACS, BioFreedom™ combined with 1-month DAPT displays significantly better efficacy and safety, with significantly lower cardiac mortality and myocardial infarction than a BMS.
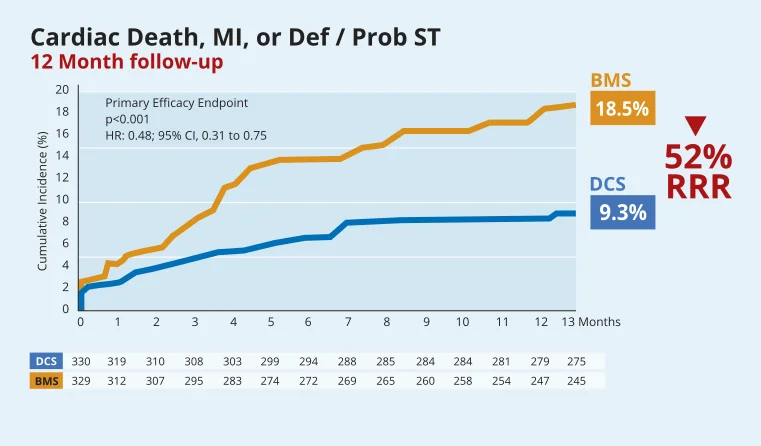
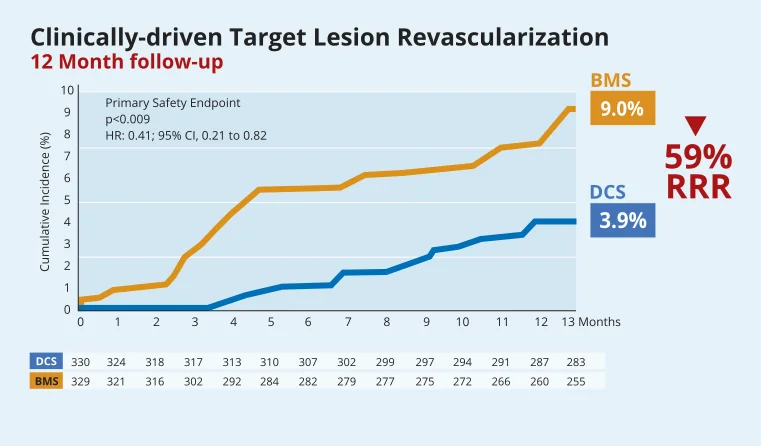
High Bleeding Risk patients: safety and efficacy benefits of BioFreedom™ Drug-Coated Stent over BMS are maintained at two years14.
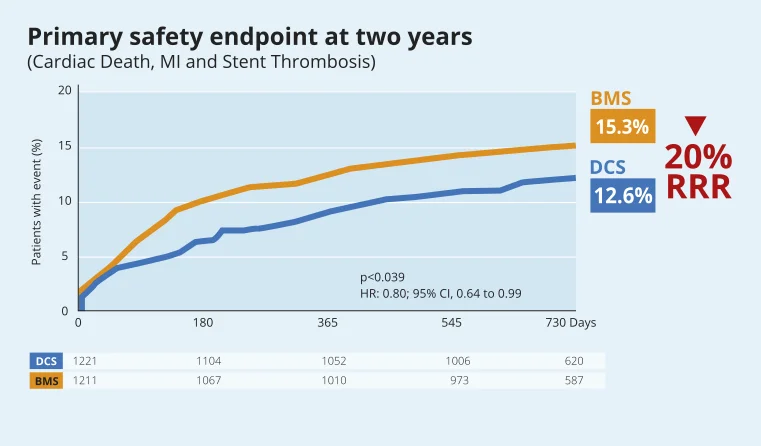
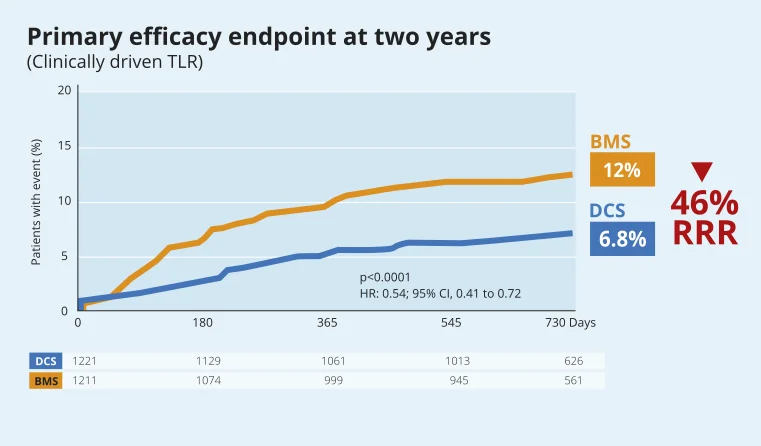
LEADERS FREE: Two-Year Outcomes of High Bleeding Risk Patients after Polymer-Free Drug-Coated stents14
BioFreedom™ is the only active stent with CE mark for ultra-short 1 month DAPT in High Bleeding Risk (HBR) patients, supported by clinical data from a double-blind randomized controlled trial.
Reproducibility of LEADERS FREE findings
> Safety of DCS with 30 day DAPT in HBR patients15
> Effectiveness of DCS with 30 day DAPT in HBR patients15
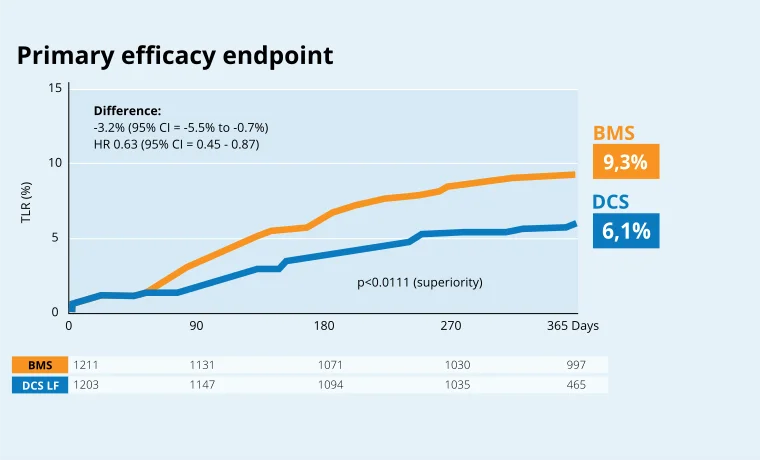
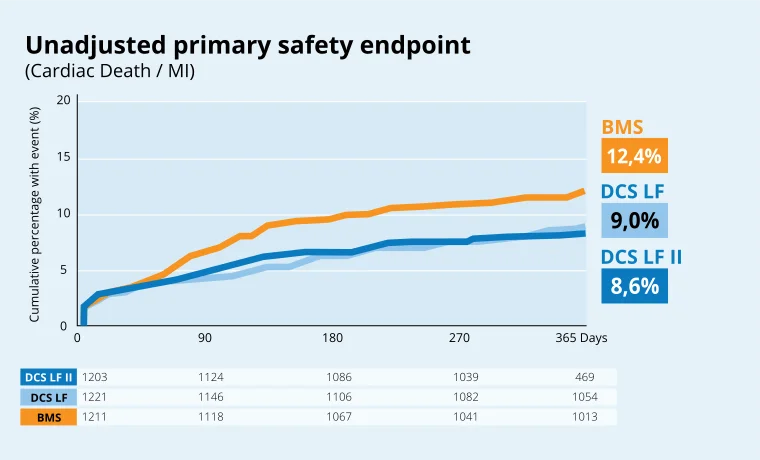
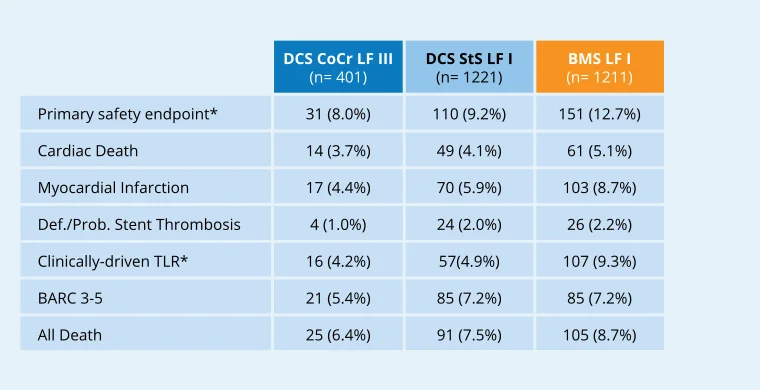
LEADERS FREE III
> CoCr DCS non-inferior to StS DCS for safety16
> CoCR DCS superior to BMS for efficacy16
11881-000-EN - Rev.04 + 11556-000-EN – Rev.01 + 11697-000-EN – Rev.01
11. Urban et al. New England Journal of Medicine 2015; published ahead of print October 14.DOI: 10.1056/NEJMoa1503943
12. European Heart Journal (2018) 39, 213–254
13. Naber et al. European Heart Journal. 2016; doi:10.1093/eurheartj/ehw203
14. Philippe Garot et al. JACC 2016. DOI: 10.1016/j.jacc.2016.10.009
15. M.W. Krucoff. Global Approach to High Bleeding Risk Patients With Polymer-Free Drug-Coated Coronary Stents: The LF II Study. Circ Cardiovasc Interv. 2020 Apr;13
16. Biolimus-A9™ coated thin strut stent in high bleeding risk patients. Oral Abstract presentation, F. Eberli, PCR eCourse 2020
* Relative Risk Reduction. Percentages are Kaplan–Meier estimates at 390 days. Presented by Philip Urban at TCT 2015. Biolimus-Coated vs. Bare-Metal Coronary Stents in High Bleeding Risk Patients
^ Rationale and Design of the LEADERS FREE Trial: A Randomized Double-Blind Comparison of the BioFreedom Drug-Coated Stent vs the Gazelle Bare Metal Stent in Patients at High Bleeding Risk Using a Short (1 Month) Course of Dual Antiplatelet Therapy Urban p. Am Heart J . 2013 May;165(5):704-9
BIOFREEDOM™ FAMILY CLINICAL PROGRAM

CAUTION: Please note that the following pages are exclusively reserved for Health Care Professionals in countries with applicable health authority product registrations. To the extent this site contains information intended for use by licensed medical professionals, such materials are not intended to offer professional medical advice. Prior to use, please consult device labeling for prescriptive information and operating instructions. Please contact your Biosensors International representative for availability or the products and registration status.
The law restricts these devices to sale by or on the order of a physician. Prior to use, it is important to read the “Instructions for Use” supplied with these devices for indications, contraindications, suggested procedures, warnings, and precautions.
Biosensors’ interventional cardiology products, including BioMatrix NeoFlex™, BioMatrix™ Alpha, BioFreedom™, BioFreedom™ Ultra, BMX-J® and RISE™ NC, are not available for sale in the United States and certain other countries. ALLEGRA™ is a product of NVT GmbH. Blue Sail Medical Co., Ltd is the ultimate parent company of NVT GmbH and Biosensors International Group, Ltd. and its subsidiaries are collaborating for the commercialization of the ALLEGRA™ device.
BioMatrix NeoFlex, BioMatrix Alpha, BioFreedom, BioFreedom Ultra, BMX-J, Juno, S-Stent and Rise NC are trademarks or registered trademarks of Biosensors International Group, Ltd. ALLEGRA is a trademark or registered trademark of NVT AG. All other cited trademarks are the property of their respective owners.