
PASSION FOR OPTIMIZED PATIENT OUTCOMES
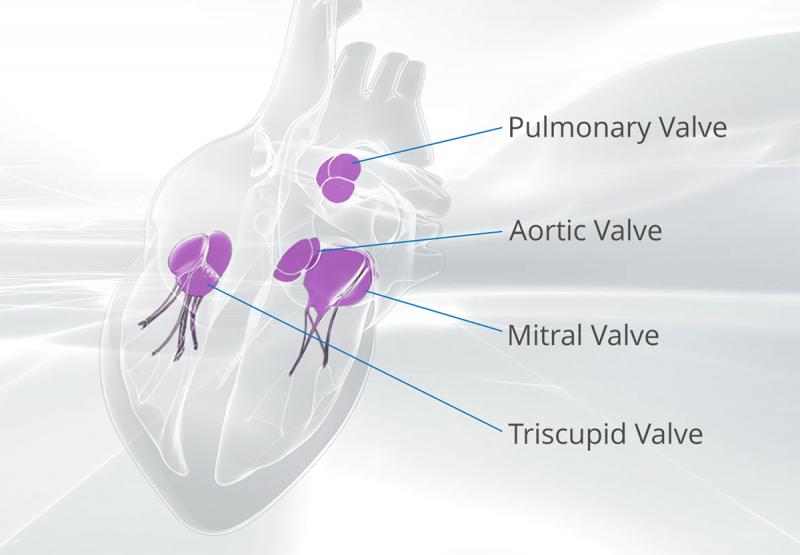
The heart is what keeps us going
It powers the body; it gives us life and energy. So does it fuel our commitment to address valvular heart disease as this can have a major impact on our wellbeing and be life-threatening.
We are passionate about learning and listening. Our success is based on working as partners with the medical and research communities. We aim to make the most of every therapeutic modality and to perfect minimally invasive cardiac valve therapies.
Those who are not able to undergo invasive surgery can then see their vitality restored and quality of life improved.

We are passionate about learning and listening
Our success is based on working as partners with the medical and research communities. We aim to make the most of every therapeutic modality and to perfect minimally invasive cardiac valve therapies.
We continuously learn from the vast surgical experience in treating valvular heart disease. We listen to physicians and engage in trusted dialogue with medical facilities to build a common understanding and nurture that passion for developing pioneering medical technology to create safe and cost-effective therapeutic approach.
11881-000-EN - Rev.04
UNCOMPROMISED HEMODYNAMICS. BY DESIGN.

ALLEGRA™ TRANSCATHETER HEART VALVE
UNCOMPROMISED HEMODYNAMICS. BY DESIGN.
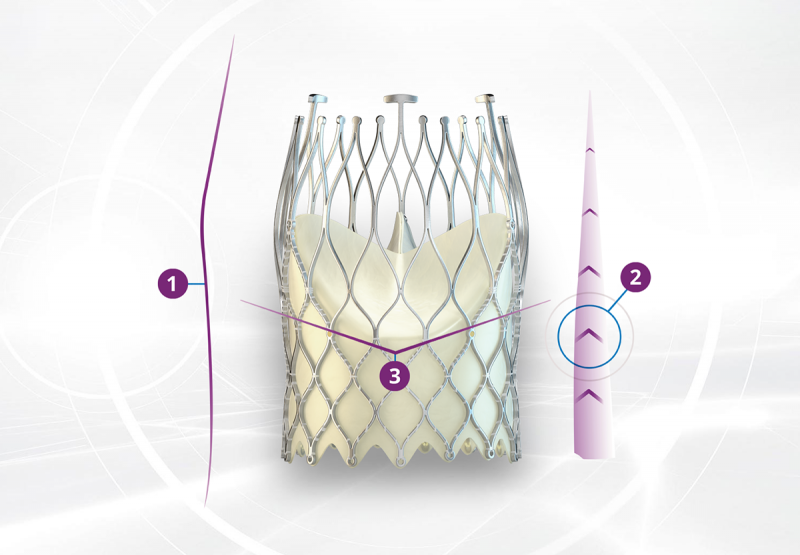
1. All about hemodynamics. The ALLEGRA™ has a unique self-expanding stent frame with convex and concave areas that provide high EOA (effective orifice areas), even in small annuli1-3. This unique stent shape also means that there is no interaction with the ascending aorta.
2. The ALLEGRA™ stent design incorporates a tailored radial force distribution with high radial force in the sealing area to achieve secure anchoring and less radial force in the outflow section. This enables a wider valve opening at the commissures and contributes to the large EOA and low mean pressure gradient1-3.
3. The ALLEGRA™ is a supra-annular valve. The new valve plane sits above the constrained and diseased native valve. This supra-annular valve position also contributes to the large EOA and low mean pressure gradient1-3.
MAXIMIZING THE EFFECT IN SMALL ANNULI
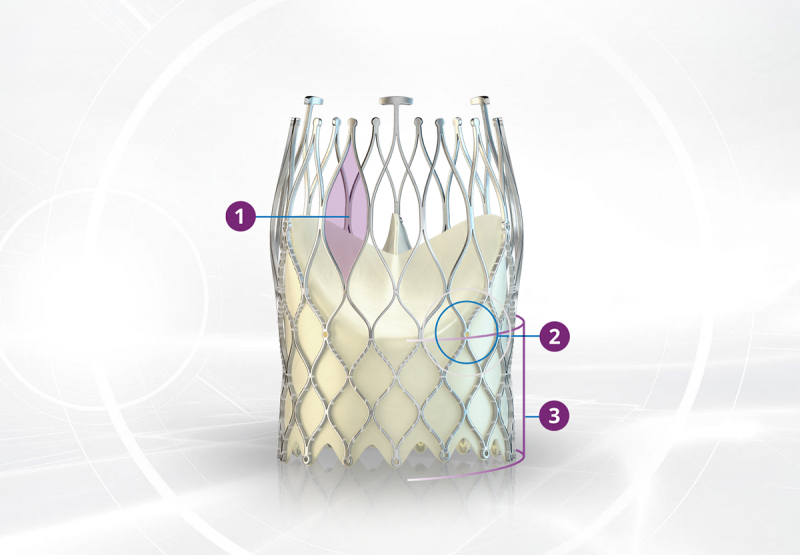
1. The design features of the ALLEGRA™ make it an excellent choice for Valve-in-Valve procedures with low frame height and unique visualization of the new valve plane by 6 radiopaque gold markers.
2. ALLEGRA™ shows better in-vitro hemodynamic performance than other TAVI devices in the Valve-in-Valve setting. In-vitro comparisons have shown higher EOA compared to other intra-annular and supra-annular valves4,5.
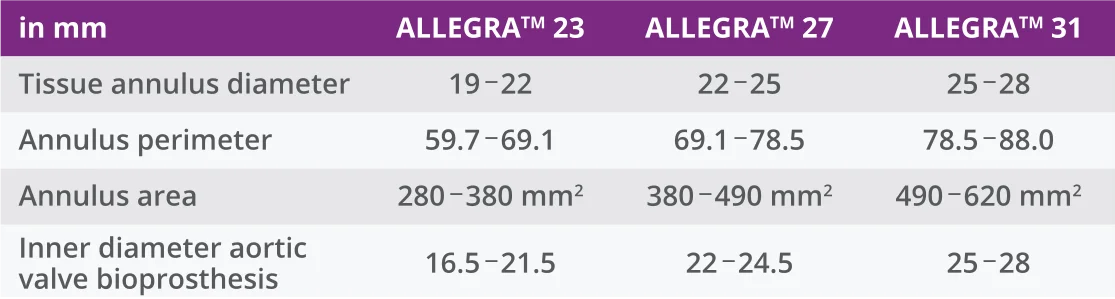
3. The ALLEGRA™ can be used with annular diameters as low as 19 mm in native valves and 16.5 mm in surgical valves. Even in these very small annuli, low single digit mean pressure gradients and high EOA can be achieved1,3.
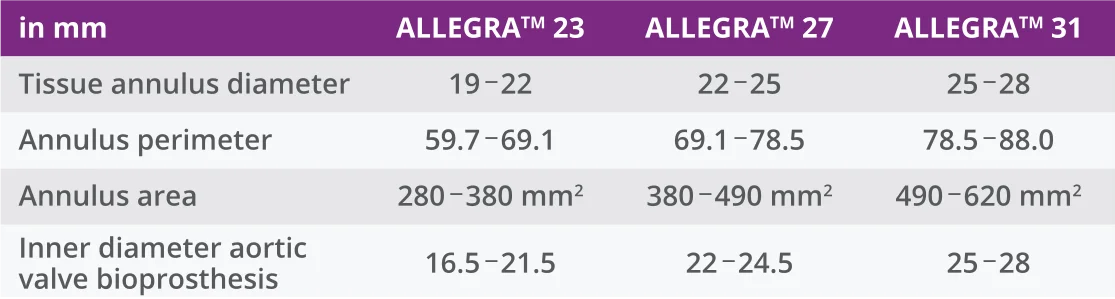
MAINTAINING CORONARY ACCESS
In patients with wide coronary sinuses, intra-annular fixation of the ALLEGRA™ and the absence of any interaction with the ascending aorta both ensure continued access to the coronary ostia.
1. In patients with narrower coronary sinuses, the outflow section of the ALLEGRA™ has large stent cells which further facilitate coronary access. The stent frame can be safely crossed with standard interventional catheters including up to 12 Fr6.
2.In all studies conducted with ALLEGRA™ so far, there is a 0% incidence of coronary obstruction1-3.
3. In patients with very low-lying coronary ostia, the 6 easily visualized gold markers which mark both the new valve plane and the top of the 12 mm high sealing skirt allow the operator to accurately position the valve taking into account the coronary heights.
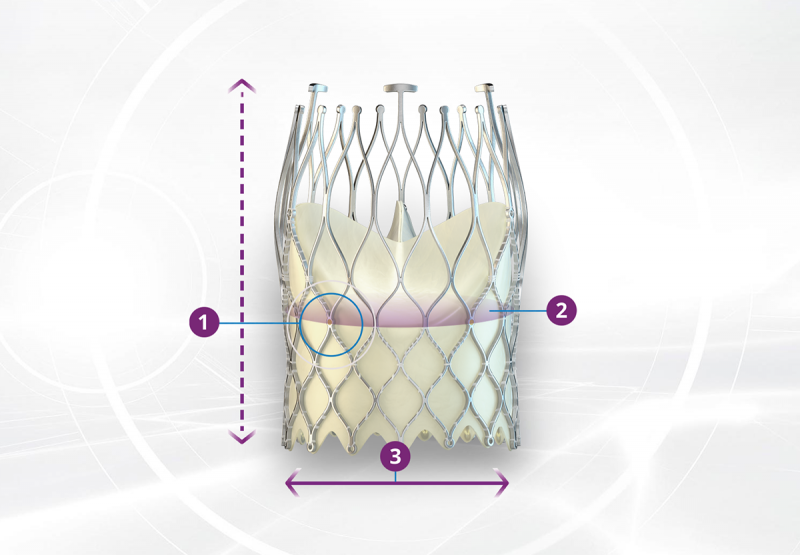
DURABILITY BY DESIGN
1. The stent frame of the ALLEGRA™ allows pole movement which reduces leaflet stress. Pole movement allows the commissural points of the leaflets to move with every cardiac cycle and reduces stress to the leaflets7. In the surgical aortic valve literature reduction of leaflet stress is associated with increased durability8.
2. For the ALLEGRA™ bovine pericardium is used for all tissue components. Bovine pericardium was chosen as it has an excellent performance profile with very good biocompatibility, outstanding hemodynamic characteristics, low complication rates and extensive data on durability from the surgical literature9.
3. The ALLEGRA™ bovine pericardium is treated to reduce calcification potential. The protective treatment is based on proven techniques such as the elimination of the phospholipid layer of the pericardial cells and the reduction of the glutaraldehyde free bonds. Both of these treatments offer anti-calcification characteristics to the pericardium.

DURABILITY BY DESIGN
MR image quality may be compromised if the area of interest is in the exact same area or relatively close to the position of the Bioprosthesis.
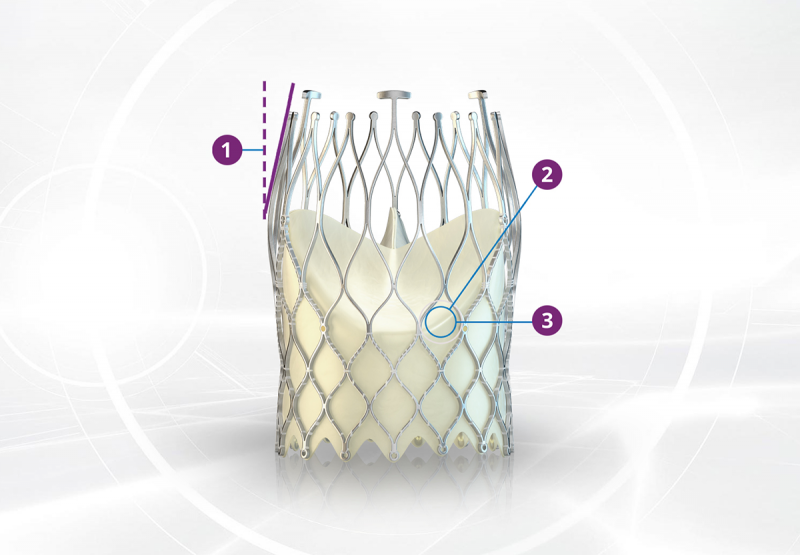
ALLEGRA™ DELIVERY SYSTEM TF
ALLEGRA™ comes with a bespoke delivery system for occlusion free Permaflow™ implantation technique.
The ALLEGRA™ delivery system with its patented Permaflow™ principle facilitates a precise and controlled release of the bioprosthesis in three steps.
1. The ALLEGRA™ delivery system offers an occlusion free Permaflow™ deployment technique with no hemodynamic compromise during implantation
2. The ALLEGRA™ delivery system incorporates an easy 3-step deployment
3. A highly flexible 15 Fr catheter with an 18 Fr valve cartridge allows enhanced cross-ability through the aortic arch
4. A user-friendly delivery handle with the unique ‘‘Squeeze-to-release’’ mechanism
5. Visibility for precise implantation is important
11881-000-EN - Rev.04
1. Wenaweser et al, Transcatheter aortic valve implantation with the NVT Allegra transcatheter heart valve system: first-in-human experience with a novel self-expanding transcatheter heart valve, EuroIntervention 2016.
2. Lemos, Clinical performance of a novel transfemoral, supra-annular, early functional, retrievable transcatheter aortic valve system”, PCR London Valves 2017 presented at Late Breaking Trials.
3. Schaefer et al, Thirty-d ay outcomes of a novel transcatheter heart valve to treat degenerated surgical valves: the VIVALL multicentre, single-arm, pilot study; EuroIntervention, 2019.
4. Data on file.
5. Sathananthan et al, Impact of implant depth on hydrodynamic function with the ALLEGRA transcatheter heart valve following valve-in-valve intervention. EuroIntervention 2019.
6. Data on file.
7. Data on file.
8. Christie GW, Barratt-Boyes BG. On stress reduction in bioprosthetic heart valve leaflets by the use of a flexible stent. Journal of cardiac surgery 1991;6:476-81.
9. Yap et al, Aortic valve replacement: is porcine or bovine valve better? 2013.
ALLEGRA is a trademark or registered trademark of NVT AG. ALLEGRA™ is CE Mark approved. Other cited trademarks are the property of their respective owners. Data on file at NVT AG for any sustained claims in this document. CAUTION: The law restricts these devices to sale by or on the order of a physician and these products are intended for the use by or under the direction of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Not available in the United States and any other country where applicable health authority product registration has not been obtained. Information contained herein only for presentation outside the US and France. ALLEGRA™ is a product of NVT AG. Blue Sail Medical Co., Ltd is the parent company of NVT AG and Biosensors Intl. and its affiliates are collaborating for the commercialization of the ALLEGRA™ device. © 2021 Biosensors Intl. Group. All rights reserved.
ALLEGRA™ FOR VALVE-IN-VALVE MAXIMIZING THE EFFECT IN SMALL ANNULI.
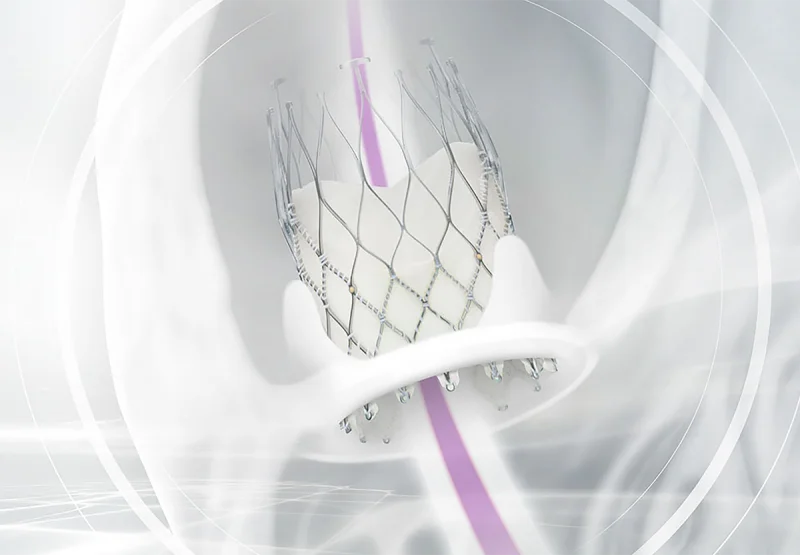
The outstanding hemodynamics of the ALLEGRA™ are particularly important in small native annuli and surgical valves (ViV).
With its supra-annular design, excellent hemodynamics and radiopaque gold markers, ALLEGRA™ is also an excellent choice for implantation into a degenerative surgical biological aortic valve.
Its dependable release mechanism facilitates straightforward, safe and effective implantation while maintaining biological circulation.
THESE FEATURES ENABLE SAFE AND EXPEDIENT IMPLANTATION ON THE FIRST ATTEMPT

Maximizing the effect in small annuli
Patients with small annuli derive particular benefit from the use of the ALLEGRA™ valve, due to the combination of wide valve opening and supra-annular valve plane. The ALLEGRA™ can be used with annular diameters as low as 19 mm in native valves and 16.5 mm in surgical valves. Even in these very small annuli, low single digit mean pressure gradients and high effective orifice areas can be achieved1,3.
Excellent choice for Valve-in-Valve procedures
The design features of the ALLEGRA™ make it an excellent choice for Valve-in-Valve procedures with low frame height and unique visualization of the new valve plane by 6 radiopaque gold markers.
Better in-vitro hemodynamic performance
ALLEGRA™ shows better in-vitro hemodynamic performance than other TAVI devices in the Valve-in-Valve setting. In-vitro comparisons have shown higher effective orifice area (EOA) compared to other intra-annular and supra-annular valves4,5.
CLINICAL OUTCOMES

11.6 ± 3.7 mmHg
The pre-procedural mean pressure gradient3 of 37.1 ± 13.8 mmHg was reduced to 11.6 ± 3.7 mmHg after the operation
0 %
total mortality
0 %
need for new pacemaker
1.4 ± 0.52 cm²
The effective opening surface increased from a baseline of 1.18 ± 0.58 cm² to 1.4 ± 0.52 cm² after 30 days
11881-000-EN - Rev.04
1. Wenaweser et al, Transcatheter aortic valve implantation with the NVT Allegra transcatheter heart valve system: first-in-human experience with a novel self-expanding transcatheter heart valve, EuroIntervention 2016.
2. Lemos, Clinical performance of a novel transfemoral, supra-annular, early functional, retrievable transcatheter aortic valve system”, PCR London Valves 2017 presented at Late Breaking Trials.
3. Schaefer et al, Thirty-d ay outcomes of a novel transcatheter heart valve to treat degenerated surgical valves: the VIVALL multicentre, single-arm, pilot study; EuroIntervention, 2019.
4. Data on file.
5. Sathananthan et al, Impact of implant depth on hydrodynamic function with the ALLEGRA transcatheter heart valve following valve-in-valve intervention. EuroIntervention 2019.
6. Data on file.
7. Data on file.
8. Christie GW, Barratt-Boyes BG. On stress reduction in bioprosthetic heart valve leaflets by the use of a flexible stent. Journal of cardiac surgery 1991;6:476-81.
9. Yap et al, Aortic valve replacement: is porcine or bovine valve better? 2013.
ALLEGRA is a trademark or registered trademark of NVT AG. ALLEGRA™ is CE Mark approved. Other cited trademarks are the property of their respective owners. Data on file at NVT AG for any sustained claims in this document.
CAUTION: The law restricts these devices to sale by or on the order of a physician and these products are intended for the use by or under the direction of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Not available in the United States and any other country where applicable health authority product registration has not been obtained. Information contained herein only for presentation outside the US and France.
ALLEGRA™ is a product of NVT AG. Blue Sail Medical Co., Ltd is the parent company of NVT AG and Biosensors Intl. and its affiliates are collaborating for the commercialization of the ALLEGRA™ device. © 2021 Biosensors Intl. Group. All rights reserved.
ALLEGRA™ INSTRUCTION FOR USE
The ALLEGRA™ TAVI System TF is a CE approved transcatheter heart valve system which is intended to treat degenerative calcified aortic valves or failing surgical aortic valve bioprostheses in a minimal invasive transcatheter implantation technique.
ALLEGRA™ Summary of Safety and Clinical Performance (SSCP) for user
Should you want to receive the SSCP of the ALLEGRA™ TAVI System TF, please use the request form below. This request will only be processed if you are a health care professional and clinical user of the device.
SSCP Request form for user:
The fields marked with * are mandatory.
11881-000-EN - Rev.04
CAUTION: Please note that the following pages are exclusively reserved for Health Care Professionals in countries with applicable health authority product registrations. To the extent this site contains information intended for use by licensed medical professionals, such materials are not intended to offer professional medical advice. Prior to use, please consult device labeling for prescriptive information and operating instructions. Please contact your Biosensors International representative for availability or the products and registration status.
The law restricts these devices to sale by or on the order of a physician. Prior to use, it is important to read the “Instructions for Use” supplied with these devices for indications, contraindications, suggested procedures, warnings, and precautions.
Biosensors’ interventional cardiology products, including BioMatrix NeoFlex™, BioMatrix™ Alpha, BioFreedom™, BioFreedom™ Ultra, BMX-J® and RISE™ NC, are not available for sale in the United States and certain other countries. ALLEGRA™ is a product of NVT GmbH. Blue Sail Medical Co., Ltd is the ultimate parent company of NVT GmbH and Biosensors International Group, Ltd. and its subsidiaries are collaborating for the commercialization of the ALLEGRA™ device.
BioMatrix NeoFlex, BioMatrix Alpha, BioFreedom, BioFreedom Ultra, BMX-J, Juno, S-Stent and Rise NC are trademarks or registered trademarks of Biosensors International Group, Ltd. ALLEGRA is a trademark or registered trademark of NVT AG. All other cited trademarks are the property of their respective owners.
